What is Passivation?Among the many processes used to prevent metal corrosion, passivation is one of the most widely used and effective. By forming a dense, protective oxide film on the metal surface, it significantly improves corrosion resistance while maintaining appearance and structural integrity. This article will delve into what passivation is, its principles, advantages, and typical applications . Whether you’re an engineer, manufacturer, or reader interested in corrosion prevention technology, you’ll find answers here and understand why passivation has become a trusted solution across industries.
What Is Passivation
Passivation is a common metal surface treatment process, typically applied to stainless steel and some alloys. Its core principle is to use an acidic solution (such as nitric acid or citric acid) to remove free iron and other impurities from the metal surface, thereby promoting the formation of a uniform, dense oxide film (primarily chromium oxide) on the metal surface . This protective film effectively isolates the metal from the external environment and enhances its corrosion resistance.
Get 20% offf
Your First Order
Passivation is not only suitable for industries with extremely high requirements for cleanliness and durability, such as aerospace, medical devices, and food processing, but also complies with international standards such as ASTM A967 and AMS 2700.
The Core Value Of Passivation
Enhanced corrosion resistance : effectively prevents rust and pitting, and improves the stability of parts in harsh environments.
Extended part life : The protective layer reduces material degradation and significantly extends the life of metal components.
Improve surface cleanliness : reduce contamination residue, make the surface smoother and easier to clean and maintain.
Comply with industry standards : Meet the requirements of international standards such as ASTM A967 and AMS 2700 to ensure that parts meet strict quality systems.
What Are The Working Principles Of Passivation
The core of passivation lies in activating the metal’s inherent protective capabilities through chemical reactions. It doesn’t artificially apply a protective layer, but rather removes surface impurities, allowing the metal to form a dense, uniform, natural oxide film that protects against environmental corrosion. Although only nanometers thick, this oxide film determines the metal’s ability to maintain long-term stability in humid, corrosive, or high-purity environments.
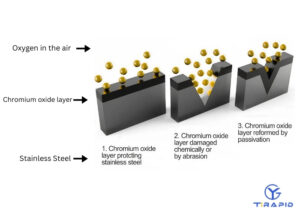
1. Surface Cleaning And Impurity Removal
During metalworking processes (such as turning, milling, and welding), free iron, cutting fluids, oils, or weld spatter often remain on the surface. These residues can damage the integrity of the natural oxide film, forming “corrosion pits.” Passivation processes typically use nitric or citric acid solutions to dissolve away free iron and impurities while minimizing damage to the substrate.
2. Formation Of Chromium-Rich Oxide Film
When the surface is cleaned, the chromium in the stainless steel reacts with oxygen in the air to form a thin film of chromium oxide (Cr₂O₃).
Thickness: Typically only 1–5 nanometers, invisible to the naked eye.
Characteristics: transparent, dense, inert.
Function: Block oxygen and moisture, inhibit further oxidation, and greatly improve corrosion resistance.
3. Function Of The Passivation Layer
Enhanced corrosion resistance: Reduces the occurrence of pitting corrosion, crevice corrosion and intergranular corrosion.
Improve surface stability: ensure that the metal remains stable in salt spray, hot and humid environments, and chemical environments.
Improved cleanability: The smooth, residue-free surface is easier to clean and meets the requirements of the pharmaceutical and food industries.
Compliance with standards: The process must comply with international standards such as ASTM A967 and AMS 2700 to ensure safe and reliable applications in medical, aviation and other fields.
Passivation can be understood as “awakening the self-repair mechanism of stainless steel”: first, surface contaminants are removed, and then the metal is allowed to freely form a protective oxide film. This is like peeling off damaged skin and then allowing new skin to recover on its own, ultimately gaining stronger protection.
What Are The Processes Of Passivation Process
Passivation is not a single action, but a set of orderly chemical and physical steps. Its core process is to thoroughly clean the metal surface, remove potential corrosion sources, and form a stable protective film in the air .
Although different manufacturers may have differences in operational details, a standardized passivation process usually includes the following main steps:
1. Cleaning
Remove oil, coolant, welding slag, and particulate matter to ensure the metal surface is fully exposed. Common methods include alkaline cleaning, ultrasonic cleaning, or solvent cleaning.
Purpose : Prevent impurities from interfering with subsequent acid treatment and ensure full contact between the acid and the substrate.
2. Acid Treatment
Immerse the parts in a nitric or citric acid solution to dissolve free iron and other impurities on the surface.
Nitric acid process: traditional method, high efficiency, but strict environmental protection and operation requirements.
Citric acid process: More environmentally friendly and safer for operators, it has become increasingly popular in recent years.
Function : Removes potential corrosion sources and creates conditions for the formation of a new passivation film.
3. Rinse
After pickling, rinse the parts thoroughly with deionized water or pure water to prevent acid residue.
Function : Avoid secondary corrosion and ensure the smooth progress of the subsequent oxidation process.
4. Drying And Natural Oxidation
After rinsing, the parts are dried and exposed to air, where a uniform, chromium-rich oxide film (Cr₂O₃) naturally forms on their surfaces.
This nanometer -thick, transparent protective film is the core achievement of passivation, significantly improving the corrosion resistance of parts, maintaining a clean appearance, and extending their service life.
Passivation vs. Other Surface Treatments
In CNC machining, parts often need to meet both high precision and high corrosion resistance requirements. Common surface treatment methods include passivation, anodizing, pickling, and electroplating. While these processes all aim to improve part performance and lifespan, their mechanisms and application ranges differ significantly. Understanding these differences can help engineers and manufacturers make the optimal choice based on their specific needs.
Passivation vs. Anodizing : Passivation is a chemical process that uses acid to remove surface impurities and allows the metal to naturally form a transparent protective oxide film. It is commonly used on stainless steel and high-nickel alloys. It barely alters the appearance and dimensions of the part, but significantly improves corrosion resistance. In contrast, anodizing is an electrochemical process, commonly used on aluminum and titanium, producing a thicker, denser oxide layer that not only resists corrosion but also provides decorative and insulating properties.
Passivation vs. Pickling : The primary purpose of pickling is to remove scale, rust, or contamination produced during welding and heat treatment, restoring the surface to its natural metallic color. It is more of a cleaning process than a protective measure. Passivation, on the other hand, further forms a protective film after surface cleaning, focusing on improving corrosion resistance.
Passivation vs. Electroplating : Passivation doesn’t add a new layer to the metal surface, but instead leverages the material’s inherent properties to form a natural protective film. Electroplating, on the other hand, deposits a metal layer (such as nickel, chromium, or zinc) onto the substrate, improving not only corrosion resistance but also appearance and, in some cases, even electrical conductivity. However, electroplating alters part size and thickness, and the process is more expensive.
What Materials Can Be Passivated
Passivation isn’t suitable for all metals. It’s primarily targeted at corrosion-resistant metals that possess the ability to self-passivate and form a stable oxide film on their surfaces. By removing free iron and impurities through acid treatment, these metals quickly develop a uniform, dense oxide layer, enhancing overall corrosion resistance. This is particularly true for stainless steel, titanium alloys, and high-nickel alloys, where the passivation process not only extends component life but also meets stringent standards in industries such as medical, food, and aerospace.
The following table summarizes commonly applicable materials and their characteristics:
| Material Category | Typical grades/examples | Features and applicable scenarios |
| Stainless Steel | 304, 316, 17-4PH | The most common passivation material, widely used in medical devices, food processing, and chemical equipment. 316 performs better in chloride corrosion resistance, while 17-4PH is commonly found in aerospace parts. |
| Titanium & Alloys | Ti-6Al-4V, etc. | It has excellent biocompatibility and is commonly used in implantable medical devices and aerospace parts. Passivation further improves corrosion resistance and surface stability. |
| High-Nickel Alloys | Inconel, Hastelloy | It performs outstandingly in high temperature and strong acid and alkali environments and is suitable for highly corrosive working conditions such as chemical and energy equipment. |
| Other Corrosion-Resistant Metals | Chromium alloy, niobium alloy, etc. | The application is relatively niche, but it has important value in special industrial environments (such as nuclear energy and deep-sea engineering). |
Advantages Of Passivation
During processing and transportation,
our products often become damaged or contaminated with free iron, leading to the risk of localized corrosion. The passivation process chemically removes these potential hazards and forms a uniform, stable protective oxide film on the metal surface, ensuring long-term reliability. Its benefits extend beyond corrosion resistance to include improved lifespan, appearance, and compliance with international standards.

The advantages of passivation span multiple dimensions: technical performance, product lifespan, cleanliness, aesthetics, and regulatory compliance. For manufacturers, these benefits translate to fewer repairs, lower maintenance costs, and higher customer satisfaction.
| advantage | illustrate | Industry Applications |
| Improve corrosion resistance | Uniformly forms an oxide film to effectively resist salt spray, moisture and chemical corrosion | Ship and aviation parts |
| Extend service life | Avoid pitting corrosion and crevice corrosion, and reduce parts scrap | Medical implants, automotive engines |
| Improve cleanliness | Removes residual iron and pollutants, reducing bacterial growth | Food processing equipment, pharmaceutical machinery |
| consistent appearance | The surface is brighter and cleaner, reducing discoloration and spots | Consumer electronics, decorative parts |
| Comply with international standards | Meet ASTM A967, AMS 2700 and other standards | Medical devices, aerospace |
Disadvantages And Limitations Of Passivation
Although passivation brings many benefits, it is not a “universal anti-corrosion solution.” It cannot fundamentally change the material properties of metals, and there are certain restrictions on the types of metals. Not all materials are suitable . Improper operation may cause excessive corrosion or cleaning residues on the surface of parts, and even put pressure on the environment. For manufacturing companies, passivation means adding additional processes and production costs, so a cost-benefit trade-off needs to be made in actual applications.
Understanding the limitations of passivation can help engineers make more scientific decisions during the design and material selection stages. When choosing whether to adopt the passivation process, companies usually consider the product’s purpose, the use environment, and customer requirements to decide whether to add this step.
| limitation | illustrate | Potential impact |
| Non-permanent protection | The passivation film may gradually degrade in high salt, high humidity and other environments | Shortened service life, requiring maintenance |
| Limited scope of application | Not very effective on metals such as carbon steel and aluminum | Special coating or other treatment is required |
| Process risks | Excessive acid corrosion or incomplete cleaning may damage parts | Affects performance and appearance |
| Increased costs | Requires additional processes and consumables, extending delivery time | Not good for mass production |
| Environmental pressure | Acid and waste liquid need to be strictly handled, increasing environmental protection costs | Impact on corporate compliance |
Best Practices For Passivation In CNC Machining
In CNC machining, parts often remain residual cutting fluid, tiny iron chips, oil stains, and heat traces after undergoing milling, turning, and drilling. These residues can easily damage the metal’s natural protective layer, exposing otherwise corrosion-resistant stainless steel or titanium alloys to risk. The significance of the passivation process lies in its “aftermath”—it’s more than just a simple pickling step, but a critical process for restoring and enhancing a metal’s corrosion resistance.
Particularly in industries like medical, food, and aerospace , parts have extremely high requirements for surface cleanliness and corrosion resistance, and even the slightest defect can pose a safety hazard. Therefore, companies implement a standardized passivation process after CNC machining to extend part lifespan while complying with international standards such as ASTM A967 and AMS 2700, enhancing product competitiveness in the global market.
Best Practices Content
Passivate Immediately After Processing
Metal surfaces are highly reactive after processing. Prolonged exposure to air or moisture can lead to secondary oxidation or corrosion. Prompt passivation minimizes the risk of contaminant adhesion and pitting corrosion.
Choose The Acid Type According To Industry Needs
Citric Acid : Environmentally friendly and safe, commonly used in medical and food processing parts.
Nitric Acid : It acts faster and is suitable for industrial and aerospace fields that require higher efficiency.
Strictly Control The Process Parameters
including acid concentration, temperature and immersion time. Too long or too high a treatment time may cause excessive corrosion, while too short a treatment time may not completely remove the free iron.
It is recommended to use deionized water or ultrapure water for thorough rinsing to avoid chloride ion and salt residue and ensure surface purity.
Complete Drying And Formation Of Uniform Oxide Film
After drying, a nano-scale chromium-rich oxide film (usually chromium oxide) will be formed on the surface, which is the final protective barrier of the passivation process.
Common Mistakes And How To Avoid Them
| Frequently Asked Questions | as a result of | How to avoid |
| Delayed passivation after processing | Pitting and rust on the surface | Passivation completed within 24 hours after processing |
| Using the wrong acid | Surface corrosion or poor performance | Select acid based on material and industry standards |
| Improper process control | Excessive corrosion or incomplete film | Strictly monitor concentration, temperature and time |
| Incomplete rinsing | Residual contaminants accelerate corrosion | Use deionized water and extend the rinse time |
| Insufficient drying | Uneven film layer and local failure | Use hot air or dry in a clean environment |
Application Industries Of Passivation
Passivation is a necessary step in many industries. As parts are increasingly used in high-precision, high-cleanliness environments and extreme environments, surface corrosion resistance, cleanliness, and lifespan directly determine product performance and safety. Passivation not only improves part appearance and consistency but, more importantly, meets the stringent standards of industries such as medical, food, and aerospace.
| industry | Typical application parts | The value of passivation |
| medical devices | Implants, surgical instruments, dental tools | Improve biocompatibility and prevent corrosion leading to infection or implant failure |
| Aerospace | Engine parts, fasteners, structural parts | Maintain corrosion resistance in extreme environments such as high temperature, high humidity, and salt spray, extending service life |
| Food processing | Pipes, valves, tanks, transmission components | Keep surfaces clean, avoid contaminants, and comply with food safety regulations |
| Semiconductors & Pharmaceuticals | Reaction vessels, transmission pipelines, high-cleanliness stainless steel components | Ensure that the surface is free of impurities, meet ultra-high cleanliness environment requirements, and avoid product contamination |
From medical and aviation to food and high-tech industries, passivation is used in nearly every field where cleanliness and corrosion resistance are crucial. It not only enhances product performance but also serves as a crucial safeguard for regulatory compliance and market access.
FAQs
What Is A Passivation Process?
Passivation Is A Controlled Chemical Treatment Where I Use Acids Like Nitric Or Citric To Remove Free Iron From Stainless Steel. This Forms A Thin, Dense Chromium Oxide Layer, Typically 2–5 Nanometers Thick, Which Enhances Corrosion Resistance. In My CNC Machining Projects, Passivation Extends Component Life By 30–50% Compared To Untreated Parts, Especially In Harsh Environments.
What Are The Disadvantages Of Passivation?
From My Experience, Passivation Is Not Permanent. The Protective Oxide Layer Can Degrade Over Time If Exposed To Chlorides Or Mechanical Wear. It Also Adds Extra Cost And Processing Time—Usually Increasing Production Costs By 5–10%. Additionally, Improper Control Of Acid Bath Conditions Can Cause Over-Etching, Residues, Or Reduced Surface Integrity, Especially On Precision Components.
What Is The Difference Between Passivation And Galvanizing?
Passivation Works By Chemically Removing Surface Contaminants And Enhancing The Natural Oxide Film On Stainless Steel, While Galvanizing Involves Coating Steel With A Zinc Layer, Typically 50–100 Microns Thick. In My Projects, Passivation Improves Cleanliness And Meets ASTM A967, Whereas Galvanizing Provides Stronger Barrier Protection But Alters Dimensional Precision, Making It Unsuitable For Tight-Tolerance Parts.
Which Chemical Is Used For Passivation?
The Most Common Chemicals I Use For Passivation Are Nitric Acid (HNO₃) And Citric Acid (C₆H₈O₇). Nitric Acid Solutions Range From 20–50% Concentration And Work Faster, While Citric Acid Is More Environmentally Friendly And Safer To Handle. Depending On The Standard, Such As ASTM A967 Or AMS 2700, I Choose The Correct Formula To Achieve Uniform Chromium Oxide Formation On Stainless Surfaces.
Will Passivation Remove Rust?
Passivation Does Not Remove Heavy Rust Or Scaling. Instead, It Removes Free Iron And Surface Contaminants That Could Lead To Future Rusting. If A Component Already Has Visible Rust, I First Use Pickling Or Mechanical Cleaning To Eliminate It, Then Apply Passivation To Prevent Recurrence. In My Practice, Passivation Extends Corrosion Resistance Up To 3–5 Times Longer Than Untreated Steel.
Conclusion
Passivation is a cost-effective, reliable surface treatment, particularly suitable for stainless steel and other high-performance alloys. It not only improves corrosion resistance and service life, but also helps companies meet stringent international quality and safety standards. It is an essential process in modern manufacturing . If you’re looking for an economical and effective surface protection solution, passivation is undoubtedly the best choice .



















